The chemical and physical composition comparison between Lab Grown Diamond and Natural Diamond
- chrislau31
- Oct 31, 2024
- 5 min read

Introduction:
In the grand tapestry of gemstones, diamonds have long held a position of unparalleled allure. However, as technology advances, a new player has entered the stage – Lab-Grown Diamonds (LGD). In this exploration, we embark on a journey into the chemical and physical realms of diamonds, comparing the intricate compositions of nature's marvels with their laboratory-crafted counterparts. Brace yourself for a scientific odyssey that not only unveils the differences but underscores the remarkable similarities that bind these diamonds in a symphony of shared brilliance.
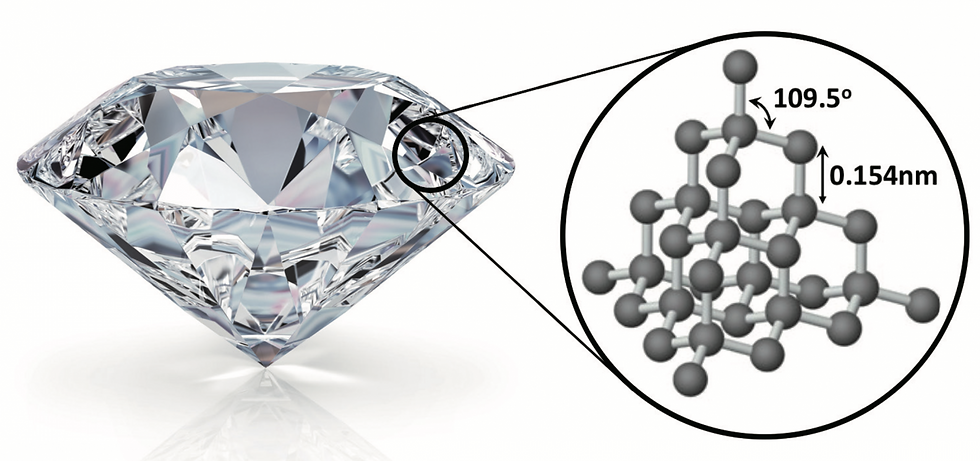
The Carbon Ballet: Atoms in Formation
Let's begin with the most fundamental building block – carbon. At the core of the enchanting world of diamonds lies the elemental foundation of carbon. Whether crafted by nature's timeless alchemy or within the confines of a laboratory, both natural diamonds and Lab-Grown Diamonds share a common composition: carbon atoms arranged meticulously in a crystal lattice structure. This arrangement is not just a testament to the elegance of simplicity but a declaration of their shared essence. In both realms, these carbon atoms form robust covalent bonds, resulting in a crystalline structure renowned for its extraordinary hardness – a signature quality that defines the enduring allure of diamonds, be they born beneath the Earth's surface or meticulously grown in a controlled environment.

Inclusions: Nature's Signature vs. Controlled Precision
One of the ways diamonds bear their unique fingerprints is through inclusions – tiny imperfections that add character. Natural diamonds can contain inclusions like minerals, gas bubbles, or other crystals, which, under the scrutiny of a skilled gemologist, offer insights into the diamond's origin and journey.
In the realm of Lab-Grown Diamonds, the story is different. The controlled environment and precision of the growth process often result in diamonds with fewer inclusions. However, it's not a complete absence. Lab-Grown Diamonds can exhibit trace elements or tiny imperfections that lend each gem its distinctive identity. The challenge for gemologists then becomes distinguishing the origin of inclusions – whether nature's spontaneous embrace or the deliberate nuances of the laboratory.
Color Palette: From Earth's Depths to Lab's Canvas
Natural diamonds display a diverse spectrum of colors, influenced by the presence of trace elements or structural irregularities during their formation. Nitrogen, for example, imparts a yellow hue, while boron may introduce shades of blue.
In the controlled environment of a lab, scientists can manipulate these factors to create a specific color palette for Lab-Grown Diamonds. Whether it's the classic transparency of a colorless diamond, the allure of fancy colors like pink or blue, or the depth of yellows and browns, the laboratory allows for a level of customization that nature, despite its artistic prowess, cannot match.

The Physical Twinship: Hardness, Density, and Thermal Conductivity
Beyond the molecular intricacies, the physical properties of diamonds contribute to their enduring allure. In terms of hardness, both natural and Lab-Grown Diamonds rank at the top of the Mohs scale, showcasing unparalleled resistance to scratches and abrasions. The Mohs Hardness Scale is used as a convenient way to help identify minerals. A mineral's hardness is a measure of its relative resistance to scratching, measured by scratching the mineral against another substance of known hardness on the Mohs Hardness Scale.
This data outline the index minerals and some common objects that are used to determine a mineral's hardness. This method is especially useful for identifying minerals in the field because you can test minerals against some very common objects (fingernail, a penny, a nail). This shared attribute underscores the inherent strength that defines diamonds, regardless of their origin.
Density, expressed as mass per unit volume, serves as a fundamental commonality between both natural and Lab-Grown Diamonds. The density of diamonds, whether formed through geological processes or synthesized in a laboratory, is approximately 3.52 grams per cubic centimeter (g/cm³). This shared density is a testament to the meticulous precision employed in laboratories to replicate the high-pressure, high-temperature conditions found in the Earth's mantle, where natural diamonds originate.
Diamonds indeed distinguish themselves in terms of thermal conductivity, standing out prominently among gemstones due to their exceptional ability to conduct heat efficiently. The thermal conductivity of diamonds, both natural and Lab-Grown, is a key property closely tied to their crystal lattice structure.
The thermal conductivity of diamonds is approximately 2,200 to 2,500 watts per meter-kelvin (W/mK). This remarkable value reflects the efficiency with which diamonds can transfer heat. The unique structure of a diamond lattice allows for efficient transmission of thermal energy through the tightly bonded carbon atoms.
In laboratory settings, the thermal conductivity of Lab-Grown Diamonds mirrors that of natural diamonds, affirming the success of replication processes. The synthesis methods, whether high-pressure, high-temperature (HPHT) or chemical vapor deposition (CVD), aim to recreate the crystal lattice structure found in natural diamond.
Refractive Index:
One of the key determinants of a diamond's brilliance is its refractive index, a measure of how much light is bent, or refracted, as it enters and exits the diamond. Lab-Grown Diamonds, engineered with precision in controlled environments, exhibit a refractive index comparable to natural diamonds. Both types of diamonds boast a high refractive index, contributing to the breathtaking play of light within the gemstone.
The refractive index of diamonds is typically around 2.42, showcasing the efficiency with which these crystals refract light. This shared characteristic underscores the successful replication of natural conditions in the laboratory, where Lab-Grown Diamonds are cultivated with a reflective brilliance akin to their naturally occurring counterparts.
Dispersion:
Dispersion, often referred to as "fire," is another captivating aspect of a diamond's brilliance. It measures the ability of a diamond to separate white light into its spectral colors. Natural and Lab-Grown Diamonds exhibit a similar dispersion, with the spectral colors dispersing into a vivid array, creating a mesmerizing display of colors within the gem.
The dispersion of diamonds is measured at approximately 0.044, showcasing the comparable ability of both Lab-Grown and natural diamonds to disperse light and create scintillating rainbows that add to their allure. This shared dispersion emphasizes the meticulous attention to detail in replicating the physical properties that make diamonds exceptional.
Nature vs. Lab: Are All Diamonds Created Equal?
The controlled environment of lab creation offers some advantages in terms of size consistency. Growing larger diamonds in nature is a gamble, subject to unpredictable conditions and inclusions that can weaken the crystal. LGDs, however, can be meticulously nurtured to reach specific sizes with greater certainty. While the current ceiling for commercially available LGDs hovers around 20 carats, research and development continue to push these boundaries. Future advancements may well see LGDs rivaling the grandiosity of their natural cousins in terms of sheer size.
But weight, of course, is not the only measure of value. Scarcity has long been a factor in natural diamonds' allure. Their formation takes millions of years, making them truly finite resources. LGDs, while not infinitely reproducible, still offer a more flexible supply chain. This can lead to more accessible pricing and wider availability, democratizing the world of diamonds for a broader audience.
Ultimately, the weight you place on weight depends on your personal priorities. If chasing record-breaking giants is your goal, natural diamonds still hold the crown. But if affordability, ethical sourcing, and a glimpse into the future of diamond creation appeal to you, LGDs offer a compelling alternative.
Remember, size is just one facet of the diamond's captivating story. Regardless of their origin or weight, both natural and lab-grown diamonds hold a unique brilliance, waiting to be discovered and cherished.
Conclusion: A Symphony of Sameness
As we explore the world of diamonds, we come to a clear conclusion – Lab-Grown Diamonds and natural diamonds are essentially the same. Although they may have different paths, they both possess incredible beauty, strength, and sparkle. The process of creating diamonds and their physical properties create a wonderful harmony, making it hard to distinguish between the two. No matter where they come from, these gems capture our hearts with their everlasting allure.
